| |
1.2- BIOMETHANOL
1.2.1- COMPOSITION
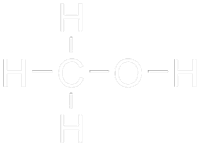
Methanol (CH3OH), which is also called methyl alcohol, is an organic, colourless, volatile, hygroscopic, flammable alcohol. It is easily biodegradable, so it results less polluting in case of spilt than most of the fossil hydrocarbons. Methanol has been traditionally synthesised from coal and natural gas, so the term "biomethanol" was created to make reference to all biomass-originated methanol, in order to avoid confusions.
This alcohol is potentially toxic for humans, since through low ingestions (around 10 ml) it produces central nervous system depression, similarly to ethanol, followed by permanent blindness caused by the damage of the optic nerve; its deadly ingestion is of around 100 ml.
During its combustion, this alcohol forms water and carbon dioxide, also emitting heat. Its fire can be extinguished with water.

1.2.2- HISTORY AND RELEVANCE
Methanol is thought to be discovered in 1661 by Robert Boyle, and its molecular identity was first established by Dumas and Peligot in 1834. Its main commercial use has always been as an industrial chemical, in order to obtain formaldehyde, dimethyl terephtalate (DMT) and biodiesel, but also as a solvent, as an ethyl alcohol denaturant and as an antifreeze additive due to its low fusion point.
At the same time, it can work as an Otto-cycle engine combustible, likewise ethanol. Its relevance as a motor fuel dates from the emblematic but tragic accident in the 1964 Indianapolis 500-mile race, where seven cars were involved in an accident that produced a great gasoline inferno of flares and smoke, and caused the death of two pilots. The United States Auto Club (USAC), knowing that one of the cars, which was fed with methanol, produced a much lighter fire (which saved its pilot's life) and burnt with nearby invisible flares so it did not reduce the visibility of the rest of the drivers, decided to promote the use of this alcohol as a race fuel. Nowadays, methanol is being compulsory used in many American motor competitions, such as Motorcycle Speedways, Monster Trucks, Champ cars and all kind of USAC races.
|
Dave Mc Donald's car. Its ignition caused themassive crash and
the death of its driver in Indianapolis.
Source: <http://www.historicmustang.com/sitebuilder/images/MacDonald_83-597x556.jpg>
|
In the field of the road transport, its representation is currently negligible in Europe, the United States or Brazil, mainly because of the ethanol competition, even though that some implantation initiatives existed in California. Nonetheless, methanol is being studied as a gasoline substitute in China since 2000's, a country that can't rely on an abundant source of oil or dedicate its alimentary crops to produce ethanol, but has got immense quantities of coal that can represent a carbon-emissive way to produce methanol.
1.2.3.- PROPRIETIES AND CHARACTERISTICS IN ENGINE. APPLICATIONS
Methanol is, similarly as ethanol, an alternative to the gasoline for internal combustion automobile engines. It can be used alone as well as blended with gasoline (in this case, those blends are named "M" plus the methanol percentage contained by the mixture: i.e., M85 for an 85% of methanol). It has a higher octane grade (113 AKI) than gasoline, but slightly lower than ethanol, and its energetic content is inferior to both of its alternatives, that is, around 15.6 MJ/litre.
These engines need an increase of the spark plug power and a higher compression rate in order to adapt the piston pressure to the higher octane grade of the methanol. The changes in materials are identical to the ethanol-based motors, because both alcohols attack the same materials (aluminium, magnesium, copper alloys, zinc and rubber principally).
Similarly to the ethanol FFVs, there are existing cars capable to run with both gasoline and methanol, detecting the methanol percentage in order to modify the pressure inside the cylinder and its spark time. However, nowadays any car company is commercialising or developing this kind of car in the occidental countries, due to the low current combustible accessibility.
Anyway, methanol can work without problems in any gasoline engine in blends up to M15, without signifying any threat to its useful life. If methanol is ever produced in a great scale, this could be the easiest way to introduce itself in the energetic market.
At last, methanol has been deeply investigated as an alternative for hydrogen as a power supply for a certain type of fuel cells. With relevant storage advantages, like a higher energetic value per volume unit and the needlessness of spending energy liquating it, methanol can be used in direct methanol fuel cells (DMFC), that produce a similar reaction to the hydrogen-powered ones, that is, the combination of H2 and O2 releasing H2O and electricity, plus the CO2 that the carbohydrate oxidation generates (see the chapter 3.3.1).
Methanol-based flexible fuel motors aren't able to run with ethanol (or vice versa) because of the distinct intrinsic conductivity of these alcohols, considering that the blend percentage detector is, normally, an electric conductivity sensor.
1.2.4- OBTAINING
Nowadays, nearby the entire commercialised methanol is produced from natural gas and coal (although it can be made from any kind of hydrocarbon) through the conversion into synthesis gas (syngas, a mixture of CO and H2) and its following conversion into alcohol using the Fischer-Tropsch process, in which those gases form methanol due to the action of a catalyst.
Anyway, this alcohol can also be synthesised from all type of biomass without exception (i.e. wood, organic waste, paper waste), unlike contemporary ethanol. Currently this way is not considered economically attractive, due to the cheapness of the alternatives; consequently, only a raise in the price of the fossil prime matter would make biomass yield as an energetic source.
In comparison to natural gas production, complex solid carbonaceous matter must follow a much longer treating path to be converted into methanol, with the consecutive energetic and economic expenses what that involves.
|
Schematic diagram of methanol production process from organic waste.
Source: HOKANSON, A. E. & ROWELL, R. M. 1977. Methanol from wood waste. [Online] Forest Products Laboratory (US Department of Agriculture), June 1977. [Cited: July 26, 2009.] <http://www.fpl.fs.fed.us/documnts/fplgtr/fplgtr12.pdf>. |
Once having the feedstock, it is necessary to partially oxidise it in the gasifier (step 1) in order to obtain a product called "crude gas", which consists in a combination of H2, CO2 and CO. To reach this oxidation, it is needed to inject air in the reaction. Crude gas passes through the scrubber (step 2) with the aim of removing organic compounds, principally acetic acid. After being cleaned, it is compressed and treated with the hot carbonate and MEA (monoethanolamine) systems (steps 4-5), both extracting residual CO2. Next, the cryogenic system (step 6) is used to separate nitrogen from the crude gas, as well as methane and other remaining hydrocarbons.
At this point, a pure syngas is obtained. Nevertheless, it is still not optimum for generating methanol, taking into consideration that the necessary compound ratio is two parts of H2 per each CO, while the actual product has a hydrogen deficit. In order to solve that, it is indispensable to obtain it from water vapour with the support of an iron catalyst (step 8) via the following reaction:

In order to remove again the residual CO2 it is convenient to repeat the hot carbonate treatment (step 11) to prepare the syngas to be transformed into methanol with the aid of a Vulcan or I.C.I. catalyst. In the reactor, an average of 95% of the syngas in converted into alcohol, and the pressure required to achieve this change is greater as the quantity of impurities increases.

Finally, the hydrocarbon mixture obtained from the reactor must be distilled (step 12) to separate the consumable pure methanol from the light ends (lower-boiling compounds of carbon-based mixture, generally butane and lighter molecules).
|
|



